Healthcare equipment providers must follow new rules for medical devices
Starting on January 1, 2025, healthcare equipment providers in the Czech Republic must follow new rules to manage medical devices. These changes, from a new law about electronic healthcare (Zákon č. 325/2021 Sb.), aim to make the system safer and more transparent. At Healtek, we understand these challenge and want to help healthcare equipment providers meet the new requirements without extensive investments.

1. Centralized registration for medical devices
According to the new law, it is necessary to create a centralized system where all medical devices must be registered before they can be sold in the Czech Republic. This system is managed by the State Institute for Drug Control (SÚKL), and vendors must ensure their products are correctly listed.
3. Regular updates of product information
Vendors must keep their product information up to date. This includes details about product availability, technical specifications, and any products that are withdrawn from the market.
2. Compliance with EU rules
Vendors must make sure that all medical devices follow European Union (EU) rules. This includes having the correct CE markings and all required documents for the product.
4. Price regulation
The Ministry of Health has introduced new rules for how medical devices are priced. Vendors need to follow these rules to make sure their prices are in line with the law.
For more information on how we can help, contact our team.
Contact HealtekHow to get ready for the changes?
Not following these rules can result in fines or being banned from selling products. It’s important for vendors to stay updated on these changes and get expert advice if needed.
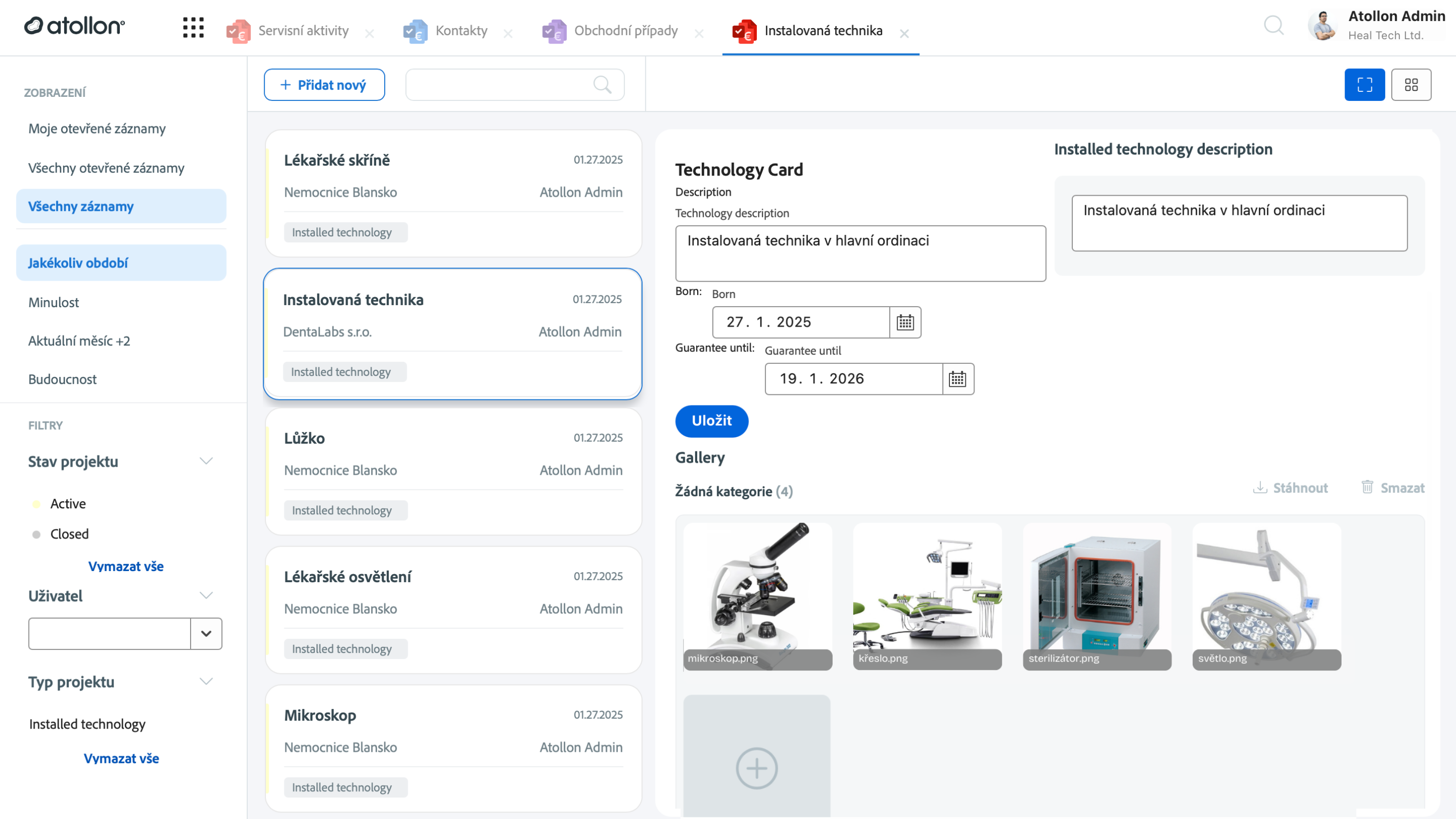
1. Centralized system integration
Keeping all equipment information in one centralized system makes it easy to access everything from sales details to service records. This approach helps improve efficiency, ensure consistency, and simplify decision-making across departments.
3. Easy product information updates
One of our recommendations for vendors is to arrange quick product information update, like availability and technical details, so it stays accurate and compliant with the law.
2. Support for EU compliance
Make sure that your products meet the European regulations, including CE marking and the necessary documents. Nowadays it is crucial to stay compliant with EU safety standards.
4. Arranging regular service activities
Make sure that all service activities are planned in advanced to not miss deadlines of regular check-ups. Try to plan it and instruct service technicians to meet EU standards.
Why compliance Is Important
These new rules help make healthcare systems safer and more efficient. At Healtek, we want to make compliance easy for you, so you don’t have to worry about fines or delays. Our solutions help save time and ensure your products meet all legal requirements.
Don’t risk being out of compliance. Let Healtek guide you through these changes, so you can focus on delivering quality healthcare.